Why collaborative storytelling can be a simple and cost-effective investment during drug development
Pharma’s cost to develop a new drug has exploded over the past decade, with estimates ranging from an industry average $2.7 billion to perhaps double that for certain mega-firms (see sidebar). In our scientific storytelling work for the industry, we notice that despite efforts to improve commercial viability by bringing a market perspective into the development stage, commercial functions like market access are still frequently unprepared to gain timely access for drugs. Every month (or year) lost in ramping up sales is actually a month (or year) peak sales sacrificed, given a limited time window for a therapy to recoup its investment. Even when an organization has implemented processes or structures to reduce this loss, endemic turnover and restructuring erode the utility of these efforts. We have found that the simple technique of collaboratively crafting a molecule’s story, however, can greatly improve launch effectiveness by ensuring the right voices are heard at the right time – ideally starting as soon as a candidate
exits pre-clinical development. This breaks down the barriers that frustrate ideal launch preparation to enable timely alignment and planning BEFORE your pivotal studies and HEOR analyses are designed.
Eroom’s Law
Whereas Moore’s Law predicts the cost of transistors will drop exponentially over time, the cost of developing a drug has increased ten-fold over the past twenty years1. The most frequently cited source for these costs, the Tufts Center for the Study of Drug Development, recently reported the cost to have risen to $2.7 billion2. That analysis, however, includes one-hit wonder biotech firms. The actual cost for Big Pharma firms – the eight that have launched more than six drugs over the past decade – can be significantly higher3.
1) Diagnosing the decline in pharmaceutical R&D efficiency. Jack W. Scannell, Alex Blanckley, Helen Boldon & Brian Warrington. Nature Reviews Drug Discovery volume11, 2012
2) Innovation in the pharmaceutical industry: New estimates of R&D costs. Joseph A.DiMasi, Henry G.Grabowski, Ronald W.Hansen. Journal of Health Economics. Volume 47, May 2016
3) The Cost Of Developing Drugs Is Insane. That Paper That Says Otherwise Is Insanely Bad. Matthew Harper, Forbes, October 16, 2017
WHAT IS SCIENTIFIC STORYTELLING?
For our customers, attention is an increasingly precious commodity. Only the most engaging science stories will cut through. If you don’t define your story, the world will do it for you. Aligning on credible, consistent and compelling science story helps connect professionals and patients with why you exist.
Your Scientific Story:
• establishes value-based differentiation
• defines value across stakeholders
• creates a shared understanding through durable, concise messages that resonate
A powerful way to create a solid story behind your franchise or molecule and to drive sustainable behavior change it is to have it co-created by key stakeholders. Development, Comms, Market Access, Marketing, Regulatory and Medical Affairs should be at the top of your list. The approach we have developed to ensure your story is compelling, consistent and credible is relatively straightforward:
Who
- Personnel selected to represent not just their functions, but key external stakeholders across critical geographies.
How
- Insight generation through internal and external interviews
- Collaborative workshopping with key stakeholders
What – “3/30/30”
- 3- second elevator pitch
- 30-second, one-page story
- 30-page deck
When
- Phase I – Target, vision and ambition
- Phase II – Molecule, unmet medical need and potential
- Phase II – Molecule, unmet medical need and potential
- Phase III – Molecule, unmet medical and emotional need and impact
- Launch – Scientific story to create springboard for commercial narrative
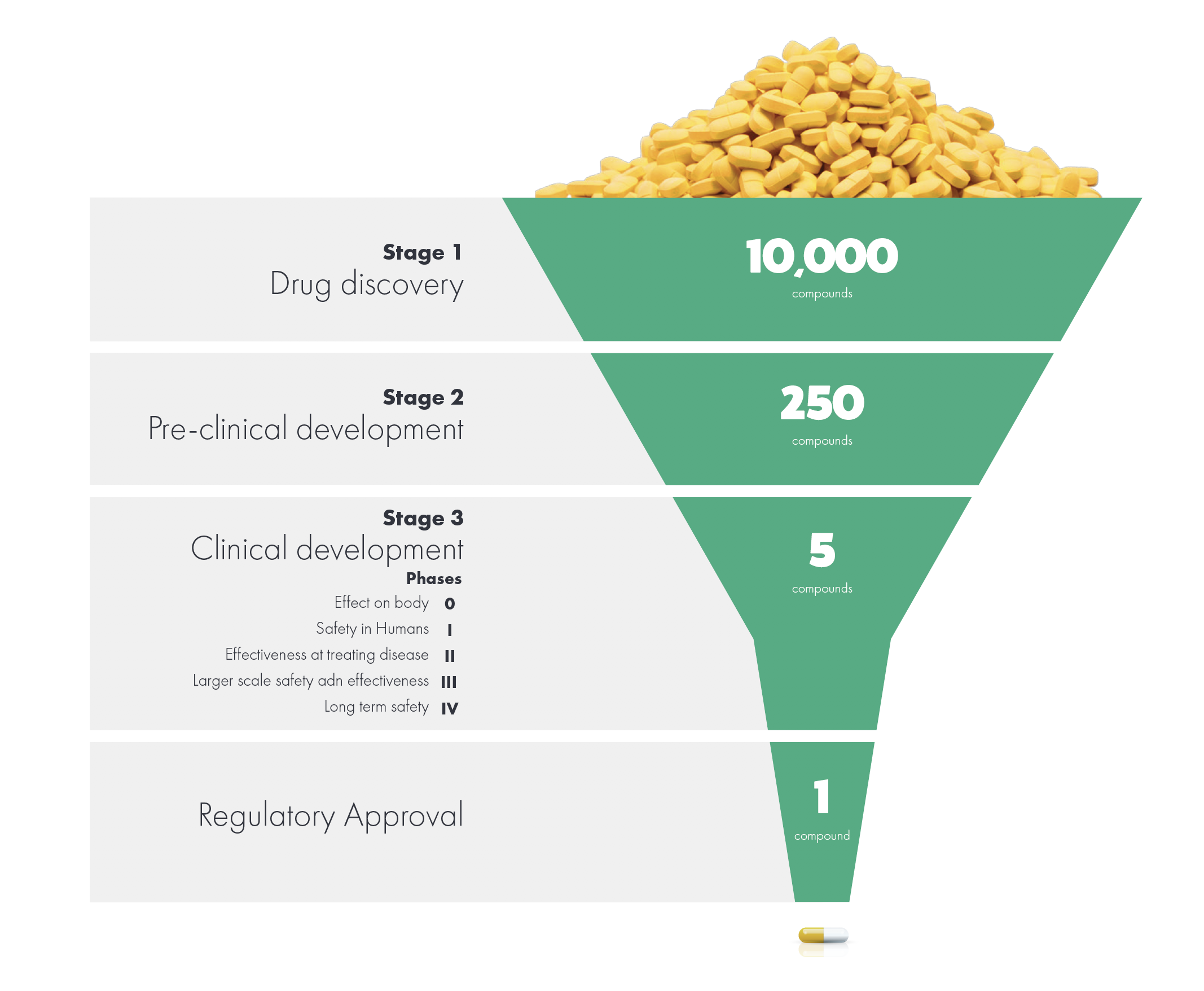
WHY IT IS IMPORTANT
For a successful launch, the appropriate voices need to be considered and acted upon in a timely fashion, BEFORE precious patent life is lost trying post-launch to do what you should have done years ago.
The TIMELY Voice of Key Stakeholders
- Voice of Customer – should be brought in by Medical and Marketing, representing patients, HCPs and key geographies
- Voice of the Enablers – can be represented by Medical Affairs, Marketing, Marketing Access. These are the partners you will need on-board to ensure the best launch possible
- Voice of the Gatekeepers – Regulatory and Market Access are critical for getting the perspectives of regulators and payors
The Matrix Maze
Many organizations have tried to ensure stakeholder perspectives are brought into the development phase, to varying degrees of success. One major hurdle is the very nature of the complex matrix environment. Most Big Pharma companies are challenged by:
- Division between R&D and Commercial
- Functional and geographical silos
- Hand-offs between teams shepherding molecules through various stages
- An organizational paradigm that is brand/molecule focused, detracting from:
- A proper portfolio perspective
- Rational, cross-brand investment
To make things harder, any processes and structure put in place to address these situations fall prey to natural turnover in personnel, exacerbated by the constant re-organization endemic to the industry.
HOW IT HELPS
Good scientific storytelling has value in itself. It creates something to rally around and guide an organization. By creating a story at the right time and in an enlightened fashion, the process itself of creation lays the groundwork for the requisite collaboration critical for launch success. In essence, the act itself of crafting a collaborative, cross-functional story creates the roadmap and conditions for coordinated activity. People and processes change rapidly these days, but a good story sticks.
WHAT’S THE PAYOFF?
The process of collaboratively crafting this story at the right time delivers a tangible kernel of stored intellectual capital – insights, messaging and planning – that can be passed like a baton to the next team. It also establishes both the culture of collaboration and the personal connections needed to support it. In today’s more fluid organizational environment, with many companies moving to an agile, “squad”-based model, a good story can also help attract resources, especially talented, engaged people. A good story developed early is like a good breakfast to start the day, or in this case, the next phase of development – before the next few billions are spent. Your molecule’s story is not going to be set in stone, but the outlines will be clear, and with them, the gaps Market Access, Clinical, and Marketing will need to to fill. You will also now have a roadmap for your scientific communications that will help guide and keep on-message your opinion leader outreach, congress activities, and publications planning. And what better way to get key stakeholders on board than to have them as writing partners?

TOM JONES
Tom is our lead consultant for the EU. His focus is on story-led business transformation, based on a quarter century’s experience as a business strategy consultant and pharmaceutical executive in the US, Latin America and Europe.